Critical Appraisal of VAERS Pharmacovigilance: Is the U.S. Vaccine Adverse Events Reporting System (VAERS) a Functioning Pharmacovigilance System?
An analysis of VAERS reporting
WHAT DOES VAERS REPORT ?
“Patterns of adverse events, or an unusually high number of adverse events reported after a particular vaccine, are called ‘signals.’ If a signal is identified through VAERS, scientist[s] may conduct further studies to find out if the signal represents an actual risk.”
CDC on Vaccine Safety
Following the initiation of the global rollout and administration of the COVID-19 vaccines1,2 on December 17, 2020, in the United States, hundreds of thousands of individuals have reported Adverse Events (AEs) using the Vaccine Adverse Events Reports System (VAERS). To date, approximately 50% of the population of the United States have received 2 doses of the COVID-19 products with 427,831 AEs reported into VAERS as of August 6th, 2021.
Pharmacovigilance (PV) is the process of collecting, monitoring, and evaluating AEs for safety signals to reduce harm to the public in the context of pharmaceutical and biological agents. Many of the issues with VAERS are becoming well known – especially with regards to reporting and recording of data – in light of the extensive use of this system this year, challenging its functionality as a pharmacovigilance system.
This appraisal assesses three issues that respond to the question of VAERS pharmacovigilance by analyzing VAERS data: 1. deleted reports, 2. delayed entry of reports and 3. recoding of Medical Dictionary for Regulatory Activities (MedDRA) terms from severe to mild. The most recently updated publicly available VAERS dataset was found to have N=1516 (0.4%) VAERS IDs removed (“missing”). Of this missing data, 13% represented death, 11% represented COVID-19 and 63% represented Severe Adverse Events (SAEs). Of these missing death data, only 59% represented redundancies – re-assigned new VAERS IDs – the remainder were unaccounted for.
A lag time between onset of AEs and entry of AEs into the VAERS public database was discovered, and it appears to depend on the AE type. For example, in the case of COVID-19 breakthrough cases, approximately mid-May, 4100 (38% of total) reports were retroactively added approximately 8.5 weeks following the original onset date. SAEs were not found to be downgraded to mild AEs (MAEs) for a tested cohort within 10 selected updates.
VAERS is designed to reveal potential early-warning risk signals from data, but if these signals are not detectable as they are received, then they are not useful as warnings. Considering the relevance of safety concerns in the face of the large numbers of AEs being reported into the VAERS system in the context of COVID-19 products, it is essential that the VAERS system be carefully and meticulously maintained. Despite the emergence of the Standard Operating Procedures (SOP) for COVID-19, VAERS is lacking in transparency and efficiency as a PV system, and it requires amendment or replacement.
Histogram plots showing distributions of the AEs of the total VAERS ID count (left) and for SAEs (right).
Histogram plot showing the distributions of the missing data of the total AE counts from the VAERS dataset according to age group.
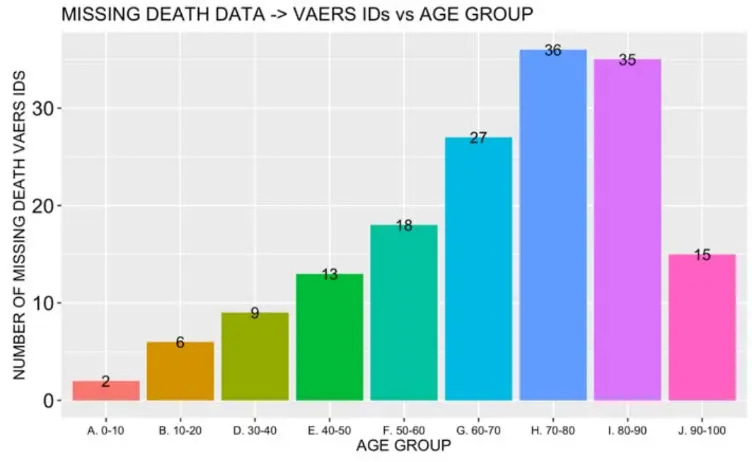
A histogram plot showing distribution of missing death data according to age group
Figure 5 shows that the distribution of deleted death data is asymmetric, unimodal and not skewed in a statistically significantly way toward any specific age group in this data set (Figure 7 (I)=0.7). Of the missing death data, 15% of reports were made within 24 hours and 28% of reports were made within 48 hours indicating a clustering of reports in very close temporal proximity to the injection.
4. Discussion
Functioning pharmacovigilance in VAERS was examined in this study. It appears from this short appraisal that although VAERS could be a functioning pharmacovigilance system, it is not being used as such. The only reference to legitimate deletion of data from the VAERS system was in the VAERS/WONDER ‘Reporting Issues’ section, which claims that ‘Duplicate event reports and/or reports determined to be false are removed from VAERS’. Despite this ‘disclaimer’, there is no way to check or validate ‘falseness’ of data that may have been removed. This means that, in the case of deleted deaths, which represent 3% of all death data, their removal needs to be explained. These deaths were reported to VAERS and recorded by hired CDC contractors. They represent people who died in temporal proximity to having been given an as-yet non-FDA-approved, experimental transfective biological product by intramuscular injection. They cannot simply be deleted. Something worth noting was the commonality in deleted entries where a causality relationship between the injections and the AE was not only implied but also suggested by the sender, which is typically the physician or emergency-room physician who attended to the individual’s case. Refer to Supplementary Table 1 for deleted death entries in the VAERS Wayback machine.
Trained contractor staff are required to enter each VAERS report into the database, and if it should be deemed necessary to delete a VAERS ID from this database once entered, then it must be documented with a valid reason for the deletion. In addition, when a VAERS ID number is changed to a new number, this should also be documented by contractor staff. It has been suggested that vaccine-induced deaths have been classified as COVID-19 deaths. If this is the case, then deaths are being skewed away from the elusive vaccine-induced death count toward the COVID-19 death count [33,34]. It is unscientific to deny any possibility that the injections are the possible cause of the injuries, particularly in some cases where the clear temporal proximity makes this possibility a high probability [8,35]. If this denial was implemented into a system of denial, it would most likely manifest in this way.
FINAL CONCLUSIONS:
It may appear that the number of missing VAERS IDs is nothing to be concerned about from an analytical point of view, but I remind the reader that these are not just data: they are people. This report addressed three issues that respond to the question of VAERS pharmacovigilance by analyzing VAERS data in relation to: 1. deleted reports, 2. delayed entry of reports, and 3. recoding of MedDRA terms from severe to mild.






While the entire story is very complex, the inaccuracies of the data indicate a more profound problem, that of the limitations of "science" in gathering and publishing data and the possible perversion of the process which leaves Americans, who generally are given choices, even those against the government's recommendations, with poor data. The panic at the beginning needed to be moderated and the claims made about the 'vaccine' were outrageous, causing the administration to treat everyone as an ignorant child. This process is ongoing with every administration, that the elites know best, a corruption of our very basic philosophy as laid out in the Declaration of Independence and executed through the Constitution.